Epidemiological studies in recent years show that in most patients, asthma and rhinitis go hand in hand, having common risk factors, the similarity of immunologic response and chronic allergic inflammation.
In foreign and domestic literature, considerable attention is devoted to the study not only the prevalence of allergic disease, namely bronchial asthma (BA), allergic rhinitis (AR), atopic dermatitis (Ad), but also the assessment of the relationship of asthma symptoms and other allergic diseases 1-5.
Among them, the primacy of the frequency of Association with AR BA belongs to 1, 4.
Studies on the prevalence of asthma and atopic dermatitis conducted in pediatric patients indicate that the so-called “atopic life cycle” and the “atopic March”, characterized by the development of various atopic diseases: in 30-60% of patients with atopic dermatitis develops BA, 35-66% – AR, with a combination of BA with AR and atopic dermatitis (dermatorespiratory syndrome) causes the most severe disease, which arise periodically exacerbation of as asthma, and comorbidities of allergic diseases with short-term remissions, resistance to medical therapy, a decrease in the quality of life of the patient. In recent years, proved a close relationship with the BA AR: 40% of patients with AR revealed BA, 80% of patients with asthma suffer from AR 9-15.
The Association of BA with AR has been an increasing effect on the bronchial tubes allergens, cold air and other triggers that exacerbate microcirculatory disorders and mucociliary dysfunction in bronchial mucosa, increase of bronchial Hyper-reactivity 16. When combined with AR allergic BA is much harder, and AR treatment reduces symptoms and facilitates a BA (level of evidence A) 17.

To study relationship between asthma and atopic dermatitis is also dedicated to a number of studies 18-20. In patients with BA has seen a high incidence of ATD, which is confirmed by a direct correlation between the frequency of asthma symptoms and atopic dermatitis according to the International study of asthma and allergies in children (International Study of Asthma and Allergy in Childhood, ISAAC) 21, 22.
As a BA, and AR, ATD share common immunopathogenetic mechanisms of formation of allergic inflammation, which are based on IgE-mediated reaction caused cause significant allergen.
Early diagnosis of allergic diseases, prevention of their development, the selection of adequate therapy to minimize symptoms, prevent disease progression and the emergence of multiple forms of Allergy is the leading in the management of patients with allergies.
Over the past decades, actively study the mechanisms of pathological reactions developing diagnostic test-systems and approaches to enhance the effectiveness of therapy. At the same time, the diagnostic search for the causal allergen is difficult in patients with polyvalent sensitization and/or cross-reactions to allergens are not genetically related groups 23, 24.
The leading etiological triggers of asthma and AR remain pollen and household allergens (A. D. ADO, V. I., Shustov, B. B. Dzantiev, 1985; vorzheva I. I., A. I. Ostroumov, 1987; I. S. Gushchin, N. I. Ilyin, S. A. Pollner, 2002; L. V. Luss, 2002; T. N. Sourovenko, 2005; T. Platts-Mills et al., 1989; N. E. Eriksson, A. Holmen, 1996; L. P. Boulet et al., 1997). Studying the spectrum of sensitization in adult patients with allergic BA (N. M. Nenashev, 2008) showed the presence of polyvalent sensitization to inhaled allergens several groups (household allergens dust mites of the family Pyroglyphidae: Dermatophagoides pteronissinus, Dermatophagoides farinae and Dermatophagoides microceras, Euroglyphus maynei; pollen – allergens-pollen of trees, grasses and weeds; epidermal; fungal), the vast majority of patients with allergic BA – 84% (CI: 80-87%).
Sensitization to mites dermatophagoides in different populations of patients with allergic BA ranges from 35% to 86% (T. N. Sourovenko, 2005; T. Platts-Mills et al. 1989; N. E. Eriksson, A. Holmen, 1996; L. P. Boulet et al., 1997). Data on the incidence of bronchial asthma in patients allergic to the pollen of plants vary in different regions of the Russian Federation from 6.5% to 76,6% (A. D. ADO, V. I., Shustov, B. B. Dzantiev, 1985; vorzheva I. I., A. I. Ostroumov, 1987; I. S. Gushchin, N. I. Ilyin, S. A. Pollner, 2002; L. V. Luss, 2002).
The presence of polyvalent sensitization to inhaled non-infectious allergens several groups (pollen, dust, epidermal) is true for patients with AR and atopic dermatitis. On average, 35% of adults with atopic dermatitis sensitized to inhaled allergens, however, these ratios vary very widely depending on the study 25, 26.
The purpose of this study was to examine the range of causal-significant allergens in allergic bronchial asthma of moderate severity, comorbid with allergic rhinitis and atopic dermatitis.
Material and methods
Surveyed 1,500 patients with BA, which was allocated among 720 patients with allergic ASTHMA, including 338 in combination with AR, 92 in combination with ATD and 132 of the patient in combination with allergic BA AR and atopic dermatitis. The age of patients (n = 132) ranged from 18 to 32 years (mean age 22,28 ± 4,99 years), among whom 83 were men (63,0%), women – 49 (37,0%). The average duration of the disease was of 11.29 ± 5,95 year. All 132 patients received basic anti-inflammatory therapy topical forms of corticosteroids, antileukotriene drugs (35.3% respectively), a blocker of H1-histamine receptors (13.3%), in line with international and national recommendations for the diagnosis and treatment of allergic ASTHMA, AR and atopic dermatitis.
The main group (group 1) were included 88 patients with allergic ASTHMA, comorbid AR and ATD (56 men and 32 women), ranging in age from 18 to 32 years (mean age 22,38 ± 0,54). The comparison group (2nd group) comprised 44 patients with allergic ASTHMA, comorbid AR and Ad (27 men and 17 women), ranging in age from 18 to 32 years (mean age of 21.48 ± 0,69) (p = 0,3094). The average age of the debut of BA patients amounted to 8,12 ± 1,47 years. The duration of allergic ASTHMA, comorbid AR and Ad ranged from 3 to 30 years, mean duration of illness of the study group (n = 132) % to 11.75 ± 5,95 year. The duration of the disease allergic ASTHMA, comorbid with AR and Ad, before the first visit to the allergist for patients of the 1st group averaged 11,18 ± 0.55 years for patients of the 2nd group of 12.32 ± 1,94 years (p = 0,3889).
Comprehensive examination included a detailed compilation of complaints, study of medical history, allergic history and life history, evaluation of factors influencing the course of the disease, physical examination. Standard laboratory studies included: General blood analysis, General urine analysis. All patients underwent the study of respiratory function by method of spirography and peakflowmetry.
To diagnose allergic diseases, identification of causally significant factors (allergen), contributing to its development, was used allergological examination methods, which included: collection allergic history, skin tests with 46 kinds of water-salt extracts of non-infectious (domestic, epidermal, pollen, food) allergen provocation tests (according to indications), immunological laboratory methods of examination (determination of the level of antigen-specific immunoglobulin E (IgE) in the serum (as indicated).
Skin testing was conducted using standardized medical diagnostic allergens containing 10,000 protein nitrogen units (PNU) in 1 ml, made of pollen, house dust, mites house dust, feather pillows, animal hair, food products have been registered and approved for use in Russia. Evaluation of the results of test carried out in 20 minutes according to the scale developed by N. D. by Beklemishev and V. With Moskvicham (1974): negative (-) reaction is not different from control, doubtful (±) – redness without blister at the site of scarification, weakly positive ( + ) is a blister with a diameter of 2-3 mm with hyperemia, positive (++) – blister up to 5 mm, slight flushing, strongly positive (+++) – blister of 10 mm with erythema and pseudopodia, very sharp degree positive (++++) reaction – blister more than 10 mm with extensive erythema and pseudopodia.
The study materials were subjected to statistical analysis using nonparametric tests. Accumulation, correction and systematization, statistical analysis of baseline information and visualization of the obtained results was conducted in Excel spreadsheets. Biometric analysis was carried out using the packages Statistica 7 and Microsoft Excel’s capabilities.
Results and discussion
All patients (n = 132) in the anamnesis there were indications clinical manifestations of airway hyperresponsiveness in case of external trigger factors. In 1/3 of patients as the trigger was made by the hot weather, the smell of cosmetic and synthetic detergents, high humidity. Reaction on frosty air were described a quarter of patients in the form of a paroxysmal dry cough. All patients (n = 132) as a specific trigger of asthma attacks was noted by contact with the causal-significant allergens: pollen – pollen of trees, grasses, weeds and household – house dust, house dust mites, feather pillows.
According to the results of skin testing with non-infectious allergens in 100% of cases positive results were obtained in the form of a combination of household and pollen sensitization.
Household sensitization was predominantly characterized by sensitivity to allergens of a house dust (100%) and dust mite (75,8%). Pollen sensitization was presented with sensitivity to tree pollen allergens (birch pollen – 95,5%, alder – 77,3), grass (pollen bonfire (76,5%), hedgehogs (79,5%), fescue (77,3%)) and weed grasses (pollen of Artemisia (97,7%), quinoa (for 90.9%)) (Fig. 1).
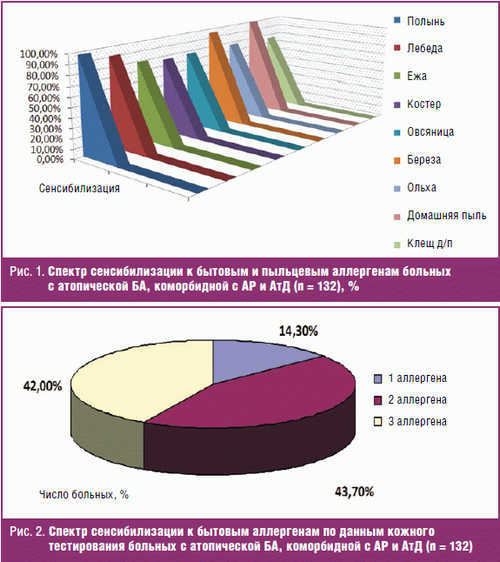
The sensitivity to allergens of house dust observed in the form of monoantibiotic (1/4 patients) and in combination with other allergens of this group (3/4 – mites household dust) (Fig. 2).
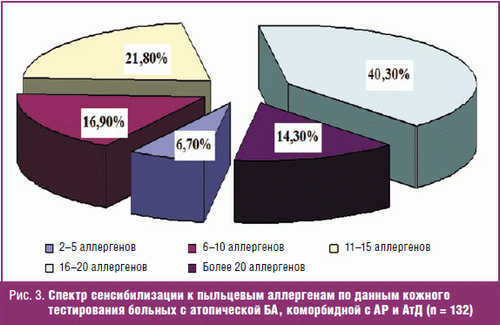
When studying the spectrum of pollen sensitization in 99.9% of the examined patients revealed high sensitivity to the 3-10 or more allergens (Fig. 3).
Some importance was given to the severity of skin tests. When testing with household and pollen allergens were recorded kozhno-allergic reactions immediate type of different intensity (from “+” to”++++”) was taken into account in the selection of allergens for conducting allergen-specific immunotherapy (ASIT).
From the analysis of the results of skin testing established a sensitivity to the pollen of weeds, and in particular to pollen allergens of Artemisia with the intensity of the “++++” in 58 (43.9 per cent) patients, “+++” 38 (28.8 per cent); quinoa – “++++” in 25 (18.9 per cent) patients and “+++” in 35 (26.5 per cent). Among the allergens of grasses cause-significant was fire (“+++” at 22.0% (29) cases “++++” – 17.4% (23)) and fescue (“+++” – 30 (22,7%) patients, “++++” – 28 (21,2%)). From tree pollen allergen hypersensitivity “+++” to the birch pollen was observed in 39 (29.5 percent) patients, “++++”, in 37 (of 28.0%).
When specific testing in addition to local registered General systemic reaction. So, 7 (5,9%) patients had intermittent attacks of dizziness, 4 (3,4%) asthma, 9 (7,6%) – nasal congestion, rhinorrhea, which correlated with the intensity of skin reactions to allergens (p < 0,0038). General reactions were stopped on their own within hours after receiving symptomatic treatment (bronchodilators, inhaled corticosteroids, antihistamines).
Thus, all 132 patients with allergic ASTHMA, comorbid with AR and Ad, set the combination, household (house dust, house dust mites) with a multivalent pollen sensitization (to the three groups of pollen allergens – pollen of trees (birch), grass (fire, fescue), and weeds (mugwort, quinoa)). Moreover, the overwhelming majority (of 85.75) patients with allergic ASTHMA, comorbid AR and atopic dermatitis, and sensitization is characterized by a combination of 2-3 of allergens and only 14.3% presented with sensitivity to one of the cause-significant factors (allergen house dust). The spectrum of sensitization to pollen allergens in these patients, according to the skin test, 94.3% is characterized by a combination of 6 and more than 20 allergens, and only 6.7% from 2 to 5 kinds of allergens.
The definition of the spectrum of cause-significant factors in the development of allergic ASTHMA, comorbid with AR and Ad, were important in the selection of ASIT.
Insights
- Monoantibiotic (sensitivity to a single allergen) in patients with allergic ASTHMA, comorbid AR and Ad not found.
- The most frequent causal significant factors in the development of allergic ASTHMA, comorbid AR and atopic dermatitis, is a combination of domestic and multivalent pollen sensitization to house dust from 100% of allergens dust mite is 75.8%, the pollen of weeds (wormwood, goose-foot) – 72,7%, tree pollen allergens (birch) – 57,5% to the pollen of grasses (fire, fescue, hedgehog) – 43.9 per cent.
Literature
- Nenasheva N. M. Bronchial asthma and related diseases: focus on allergic rhinitis // Practical Oncology. 2014. No. 1. S. 2-8.
- Gough H. et al. Multi morbidity of Allergic asthma, rhinitis, and eczema over 20 years in the German MAS birth cohort // Pediatr. Allergy Immunol. 2015. Vol. 22.
- Henriksen L. et al. Incidence rates of atopic dermatitis, asthma, and allergic rhinoconjunctivitis in Danish and Swedish children // J. Allergy Clin. Immunol. 2015. Vol. 136, No. 2. P. 360-366.
- G. Brozek et al. Increasing prevalence of asthma, respiratory symptoms and allergic diseases: Four repeated surveys from 1993-2014 // Respir Med. 2015. Vol. 109, No. 8. P. 982-990.
- Garcia-Aymerich, J. et al. Phenotyping asthma, rhinitis and eczema in MeDALL population-based birth cohorts: an allergic comorbidity cluster // Allergy. 2015. Vol. 70, No. 8. P. 973-984.
- Emelyanov A.V. and other Allergic rhinitis and asthma in real clinical practice: results of the Russian multicenter study // Russian allergological journal. 2012. No. 1. P. 29-36..
- Chichkova N. V., Lopatin A. S., Gitel E. P., Suleymanov N. C. Assessment of the immune system in patients with bronchial asthma with concomitant diseases of the nasal cavity and paranasal sinuses // Vestnik otolaryngology. 2012. No. 12. S. 27-30.
- Bottema, R. W., Nolte I. M., Howard T. D. et al. Interleukin 13 and interleukin 4 receptor-alpha polymorphisms in rhinitis and asthma // Int. Arch. Allergy Immunol. 2010. Vol. 153, No. 3. P. 259-267.
- Kungurov N. V., Gerasimova N. V., Kokhan M. M. Atopic dermatitis. Types of flow, principles of therapy. Ekaterinburg, 2000. 265 C.
- Sergeev Yu. V., Novikov D. K., Karaulov A. V., Sergeev A. Yu. Atopic dermatitis: clinical heterogeneity of forms and diversity of mechanisms of pathogenesis // Immunopathol. Allergol. Infector. 2001. No. 3. P. 61-73.
- Papadopoulos N. G., Arakawa H., Carlsen K. H., Custovic A. et al. International consensus on (ICON) pediatric asthma // Allergy. 2012; 67 (8): 976-997.
- W. C. Moore, D. A. Meyers, S. E. Wenzel et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program // Am J Respir Crit Care Med. 2010; 181: 315-323.
- Howrylak, J. A., Fuhlbrigge A. L., Strunk, R. C., Zeiger, R. S., Weiss S. T., Raby, B. A. Childhood Asthma Management Program Research Group Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications // J Allergy Clin Immunol. 2014; 133 (5): 1289-300, 1300. e1–12.
- Nazarova E. V., Latysheva E. A. New horizons in the treatment of bronchial asthma: Relvar of Ellipta – innovative drug in an improved delivery system // RAGE. 2015; No. 5, 82-89.
- The national program “Bronchial asthma in children. Strategy of treatment and prevention.” 4-e Izd. M., 2012. 180 C.
- Guseva E. D., Aref’eva N. A. Immunobiologicheskie indicators in children with allergic rhinitis and bronchial asthma // Russian rhinology. 2010. No. 3. P.7.
- Brozek J. L., Baena-Cagnani C. E., Bonini S. et al. Methodology for development of the allergic rhinitis and its impact of asthma Guideline 2008 update // Allergy. 2008. Vol. 63, No. 1. P. 38-46.
- Revyakina V. A., Filatova T. A. From atopic dermatitis to bronchial asthma in children // Attending Physician. 2006. No. 1. P. 16-20.
- Galli E. et al. Atopic dermatitis and asthma // Allergy Asthma Proc. 2007. Vol. 28, No. 5. P. 540-543.
- Foliaki S. et al. Prevalence of symptoms of childhood asthma, allergic rhinoconjunctivitis and eczema in the Pacific: the International Study of Asthma and Allergies in Childhood (ISAAC) // Allergy. 2007. Vol. 62, No. 3. P. 259–264.
- Wang H.-Y. et al. Disparate geographic prevalence of asthma, allergic rhinoconjunctivitis and atopic eczema among adolescents in five Canadian cities // Pediatric Allergy and Immunology. 2010. Vol. 21, No. 5. P. 867-877.
- M. Spergel J. Epidemiology of atopic dermatitis and atopic march in children // Immunol // Allergy Clin. North Am. 2010. No. 30. P. 269-280.
- M. Osterballe, T. K. Hansen, C. G. Mortz et al. The prevalence of food hypersensitivity in an unselected population of children and adults // Pediatr Allergy Immunol. 2005. 16: 567-573.
- Bousquet J., Gern J. E., Martinez F. D. et al. Birth cohorts in asthma and allergic diseases: report of a NIAID/NHLBI/MeDALL joint workshop // J Allergy Clin Immunol. 2014. 133 (6): 1535-46.
- Eller, E., Kjaer, H. F., Host, A. et al. Food allergy and food sensitization in early childhood: results from the DARC cohort // Allergy. 2009. 64: 1023.
- Flohr C., Johansson S. G., Wahlgren C. F., Williams H. How atopic is atopic dermatitis? // J Allergy Clin Immunol. 2004. 114: 150.